New Tumor Treatment for Malignant Pleural Mesothelioma Approved by FDA
In May 2019, the Food and Drug Administration approved a device for the treatment of malignant pleural mesothelioma under the Humanitarian Use device section of their policy on rare diseases.
What is the new treatment?
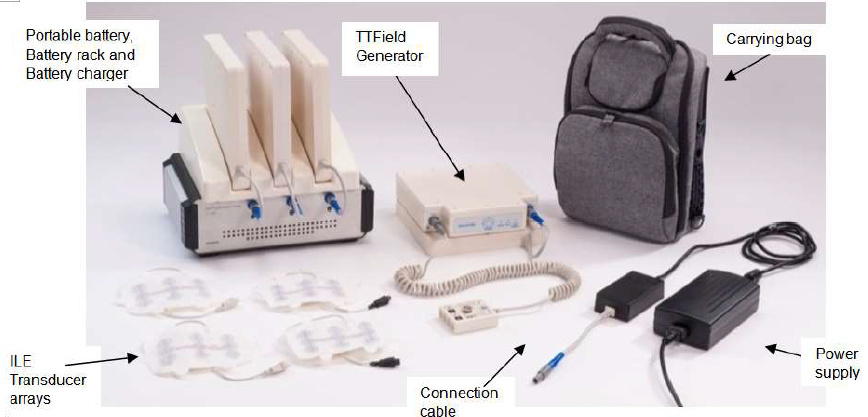
This new therapy is Novacures’s Tumor Treating Fields delivery system, or NovoTTF-100L. Tumor treating fields are mild electric fields that pulse through the skin or scalp and interrupt cancer cells’ ability to divide. Electrodes are attached to the skin and attached to a portable device. The person keeps the portable device with them as they go about their activities of daily living. The treatment is portable and does not need to be done in a hospital or clinic setting. The therapy is continuous but can be interrupted for short time everyday. It is advised to be attached 18-20 hours a day to the person.
This treatment is non invasive and does not cause side effects that other treatments might, such as nausea, vomiting, diarrhea, or fatigue. The tumor treating fields work by acting upon rapidly dividing cells of cancerous tumors without affecting normal cells. It targets highly charged proteins in the cells. The proteins are essential to the process of cell division which is how the tumor grows and spreads. With the tumor treating fields device the tumor is then prevented from dividing and growing.
Why was it approved?
Malignant mesothelioma is a rare disease and thus fits the description for approval under the Humanitarian Use Device clause. In order to qualify for this category the FDA must determine that the medical device is intended to benefit patients in the treatment of a disease that affects or is manifested in not more than 8,000 individuals in the United States per year.
The last relevant treatment approved by the FDA was chemotherapy with Pemextred and Altima in 2004.
How was it approved?
The Tumor Treating Fields device was first approved in 2011 for treatment of aggressive brain tumors called glioblastomas. For malignant pleural mesothelioma a clinical trial called STELLAR was conducted in Europe using this technology with patients that have the disease. The results were positive with an increase of overall survival in patients who took part in the trial, all of whom were not surgical candidates. It was used in conjunction with the approved chemotherapy for malignant mesothelioma, Pemextred and cisplatin.
What does it all mean?
This is progress for some patients with malignant pleural mesothelioma. It gives them another option. This is not a cure, but an additional treatment to help overall survival used in conjunction with the first line chemotherapy, pemetrexed plus cisplatin.
When meeting with your mesothelioma expert and team ask if this treatment option would be beneficial for you or your loved one. If you have been diagnosed and are seeking information or justice, please reach out to Belluck & Fox for a free consultation.