Dedicated Invokana Exposure Attorneys in New York - We will fight for your rights

Type 2 diabetes is a known killer, but diabetics seeking help didn’t know that a leading medication for the condition poses serious risks, including death and amputation, until the Food and Drug Administration made the manufacturer issue warnings.
The label now warns of dire risks, and the warnings are justified by outcomes that have spurred hundreds of lawsuits against the manufacturer, Janssen Pharmaceuticals. Many plaintiffs allege the company rushed a dangerous drug to market and did not adequately warn the public about the risks.
Pharmaceutical companies long have been found guilty of putting profit over the safety of consumers. The billions of dollars these drugs bring in often fund misleading advertising that boosts sales. Corporate lawyers fight to keep the drugs on the shelves and warnings off the labels while battling victims who are seeking financial compensation for physical damage.
At Belluck & Fox, our nationally recognized product liability lawyers have the resources, experience and fierce determination it takes to beat pharmaceutical companies that play this dirty game. If you or someone close to you is suffering due to the serious side effects of Invokana, schedule a free consultation to learn what our law firm can do to demand the financial compensation you deserve.
What Is Invokana?
Table of Contents
Sodium-glucose cotransporter-2 inhibitors are a relatively new class of medication for Type 2 diabetes, and Invokana was the first to hit the U.S. market. SGLT2 is a protein key to the kidneys’ process of reabsorbing sugar. Invokana and other drugs with the active ingredient canagliflozin reduce glucose levels in humans by inhibiting that reabsorption.
Mitsubishi Tanabe Pharma developed Invokana in 2012, and the Johnson & Johnson subsidiary Janssen Pharmaceuticals got FDA approval in 2013 to produce and market the drug. Invokana revenue hit $586 million in 2014 and soared to $1.3 billion in 2015. Competition has taken the edge off that, but Invokana remains a huge moneymaker.
Invokana Side Effects
Type 2 diabetes is a life-altering and potentially life-ending condition. Used correctly, the right medicine can be a lifesaver. The research that typically precedes that medicine’s approval and marketing is designed to raise red flags to inform consumers if there are risks and prepare them should those risks be realized, thus saving lives and minimizing suffering.
Established side effects resulting from the use of Invokana are:
Ketoacidosis: This buildup of acids in the body can result in coma or death and can produce symptoms such as abnormal thirst, frequent urination, nausea, abdominal pain, fatigue, shortness of breath and confusion.
Amputation: Lower-limb infections, gangrene and foot ulcers can occur, leading to amputation.
Severe kidney damage: Contact your doctor immediately if you experience a loss of appetite or inability to eat or are at risk of dehydration due to vomiting or diarrhea.
Hyperkalemia: This is a high level of potassium in the blood, and it can produce abnormal heart rhythms.
Urosepsis and pyelonephritis: These serious infections can originate with urinary tract infections and can cause organ failure and death. Invokana users are at greater risk of UTIs. UTI symptoms include burning while urinating, a frequent/urgent need to urinate, abdominal pain, blood in the urine, high fever, back pain, nausea and vomiting.
Hypotension: Low blood pressure is a risk, particularly among people with kidney problems.
Hypoglycemia: Symptoms of low blood sugar include headache, drowsiness, weakness, dizziness, confusion, irritability, hunger, fast heartbeat, sweating, shaking, feeling jittery.
Yeast infections: These have occurred in men and women taking Invokana.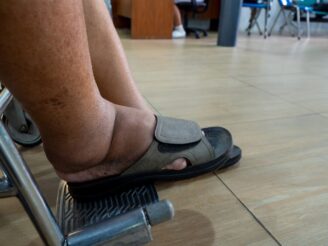
Broken bones: An increased risk of fractures and bone mineral density have been reported.
Hypersensitivity reactions: These include angioedema (swelling that often is associated with hives) and anaphylaxis (a severe and potentially lethal allergic reaction).
Cholesterol problems: Invokana has been linked to increases in low-density lipoprotein, often referred to as the bad cholesterol.
FDA Warnings About Invokana
Invokana carries the FDA’s mandatory black-box warning. “Black-box” is a reference to the box that surrounds the mandatory warning on the label. With Invokana, users are alerted that there is a significantly heightened risk of lower-leg amputation.
Here is the timeline of FDA warnings and advisories on Invokana:
- May 15, 2015: The FDA warns that SGLT2 inhibitors, including canagliflozin, can cause ketoacidosis.
- Sept. 10, 2015: The FDA issues a drug safety communication (DSC) on canagliflozin and the potential for bone fractures and decreased bone density in users. This DSC repeated the fracture warning in existing FDA data and added the bone density information.
- Dec. 4, 2015: A drug safety communication indicates the FDA will require new warnings on labels regarding elevated blood acid levels and severe urinary tract infections leading to potentially deadly complications.
- June 29, 2016: The FDA reports new findings supporting concerns that canagliflozin heightens the risk of bone fractures, though the risk is said to decrease as treatment continues.
- May 16, 2017: The FDA concludes that canagliflozin increases the risk of lower-leg amputations and orders Janssen to put a black-box warning to that effect on the label.
On its Invokana data page, the FDA offers warnings to “specific populations”:
- Pregnant women taking Invokana are putting the fetus at risk, especially after the first trimester.
- Women should not use Invokana if they are breastfeeding.
- Geriatric users could suffer from reduced blood volume.
- People with reduced blood volume and renal function are more likely to suffer adverse reactions to the drug.
- Use of Invokana is not recommended for people with severe liver problems.
Doctors and patients weigh the risks drugs pose against the benefits they can bring, and the benefits often are nothing short of miraculous. When death and injury do result, though, consumers have a right to demand compensation from the drug makers.
Invokana Lawsuits – There Are Hundreds
Plaintiffs’ complaints in the roughly 1,000 cases against Invokana accuse the defendant of a wide range of failures. These include:
- Inadequate or nonexistent warnings
- Deceptively advertising a defective drug
- Downplaying the risks of a drug
- Intentionally deceiving patients and caregivers
- Inadequate testing during a rush to market
- Negligence
As of this writing, cases were pending in state and federal courts, and cases were being consolidated in multidistrict litigation in New Jersey.
The multidistrict actions typically are guided by what are called bellwether cases that are chosen because they are representative. The first of 12 bellwethers was set to be heard in September 2018. Six of the bellwethers involved ketoacidosis, and six involved ketoacidosis with kidney damage.
Drug warnings are a matter of common sense and decency, but they also can inoculate the drug company against lawsuits involving the focus of the warning. If you or a family member has been harmed by taking Invokana, do not delay in seeking the legal help you need. Time is limited.
Have You Been Harmed by Invokana? Belluck & Fox Can Help
With more than 20 years of experience taking on large corporations in cases involving dangerous drugs and other products, the dedicated New York personal injury attorneys at Belluck & Fox have earned a reputation for aggressive advocacy. Our skilled legal team has the resources and the manpower to investigate dangerous drug claims, document your injuries, and demand the maximum compensation you deserve.
Schedule a free consultation with our nationally recognized product liability attorneys today to discuss your claim and learn about your legal options.
